Model systems
We use two model systems for our work (i) Drosophila photoreceptors where we study multiple aspects of PI signaling in the control of sub-cellular organization and and PLC based signal transduction. The goal is to discover key principles of signal transduction that are likely to be conserved during evolution but are experimentally more tractable in Drosophila. It is hoped that in the medium term, our analysis in Drosophila will inform studies of equivalent signaling pathways in mammalian models with more immediate biomedical relevance. (ii) Human disease models of brain disorders where we use a combination of human genetics and induced pluripotent stem cell (hiPSC) technology to uncover the cell biology of human brain development and function and its regulation by PIs.
Selected publications
- Raghu, P., Yadav, S and Mallampati, N. Lipid signalling in Drosophila photoreceptors. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids. Vesicular Transport. 2012; 1821(8):1154-65
- Raghu P, Joseph A, Krishnan H, Singh P and Saha S. Phosphoinositides; regulators of nervous system function in health and disease. Fron. Mol. Neurosci. 2019 Aug 29;https://doi.org/10.3389/fnmol.2019.00208
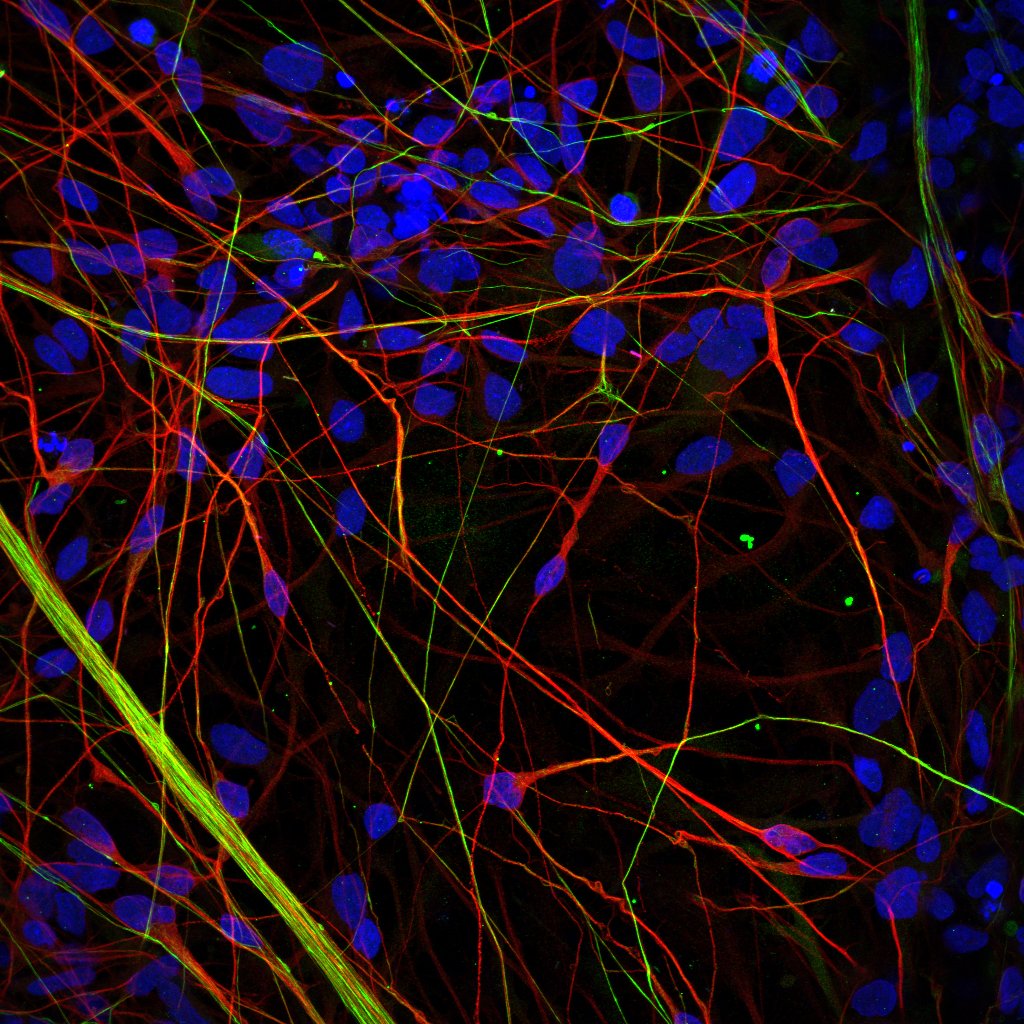
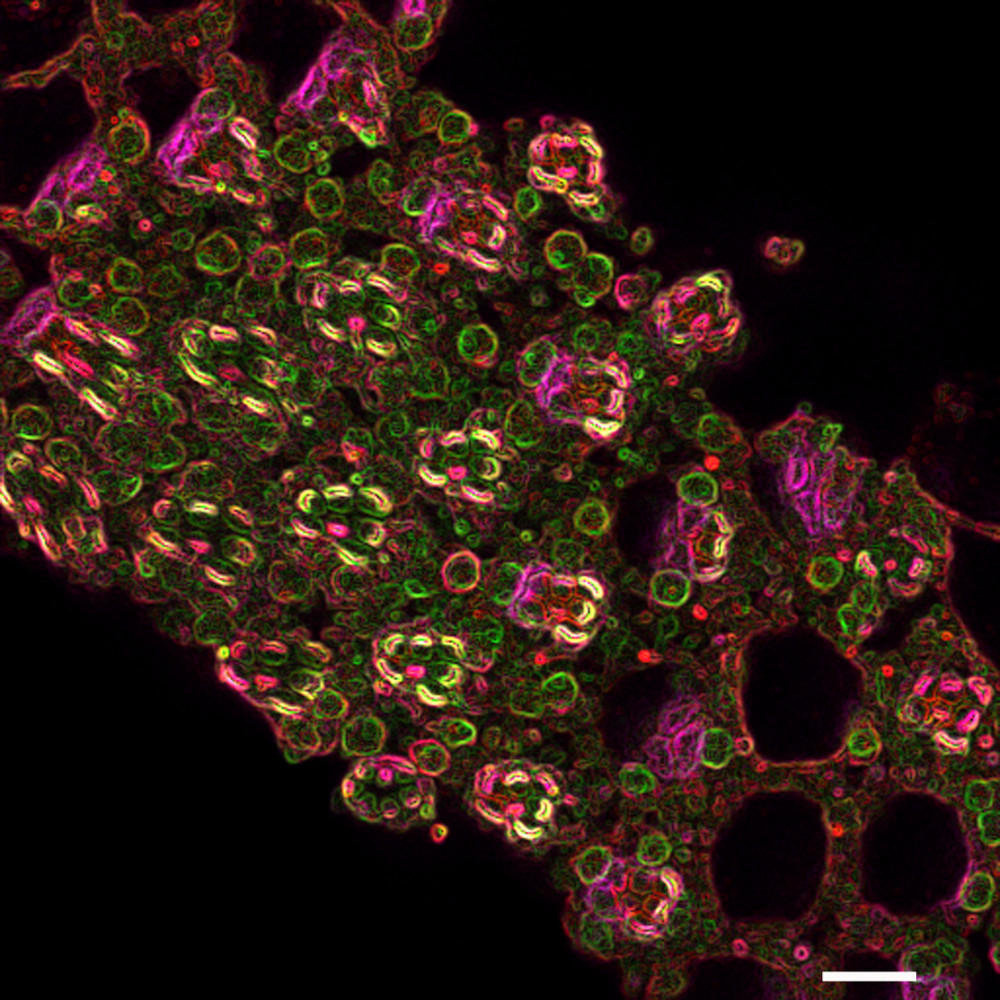
Regulation of PI(4,5)P2 signaling in neural cells
Neural cell use phospholipase C (PLC) activation and the hydrolysis of PI(4,5)P2 as a key signaling mechanism to encode information transfer. Many G-protein couple receptors important for brain development an (e.g. metabotropic glutamate receptors) use PLC mediated signaling for key processes such as learning and memory. The final output of this pathway, calcium influx is key to encode many types of information in neurons. We are interested in understanding the control of PI(4,5)P2 turnover following PLC activation. For this we use Drosophila photoreceptors as a model system (Yadav et.al). A related interest is the mechanism of action of Li, a key-mood stabilization therapy used in the treatment of bipolar disorder in human patients. The mechanism by which Li acts on the brain remains unclear. We are working on the mechanism of action of Li using a combination of human genetics, biochemistry and gene editing technologies using human iPSC derived “disease in a dish “models of human brain cells.
Selected Publications
- Control of diverse sub-cellular processes by a single multi-functional lipid phosphatidylinositol 4,5 bisphosphate [PI(4,5)P2]. Kolay, S., Basu, U and Raghu, P. Biochemical Journal. (2016) Jun 15;473(12):1681-92. doi: 10.1042/BCJ20160069.
- Balakrishnan SS, Basu U, Shinde D, Thakur R, Jaiswal M, Raghu P. Regulation of PI4P levels by PI4KIIIα during G-protein coupled PLC signaling in Drosophila photoreceptors. J Cell Sci. 2018 Jul 6. pii: jcs.217257. doi: 10.1242/jcs.217257. ADBS consortium paper (P. Raghu member of consortium).
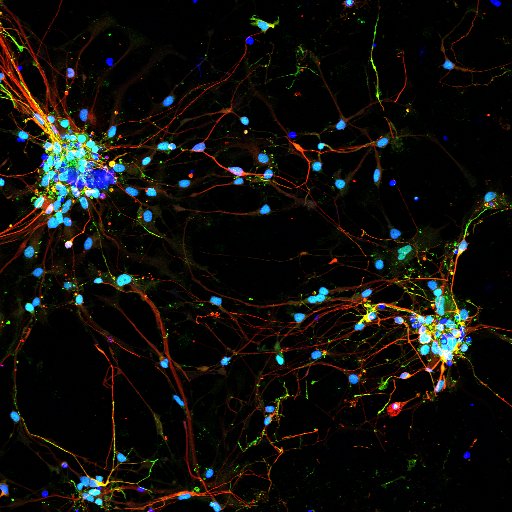
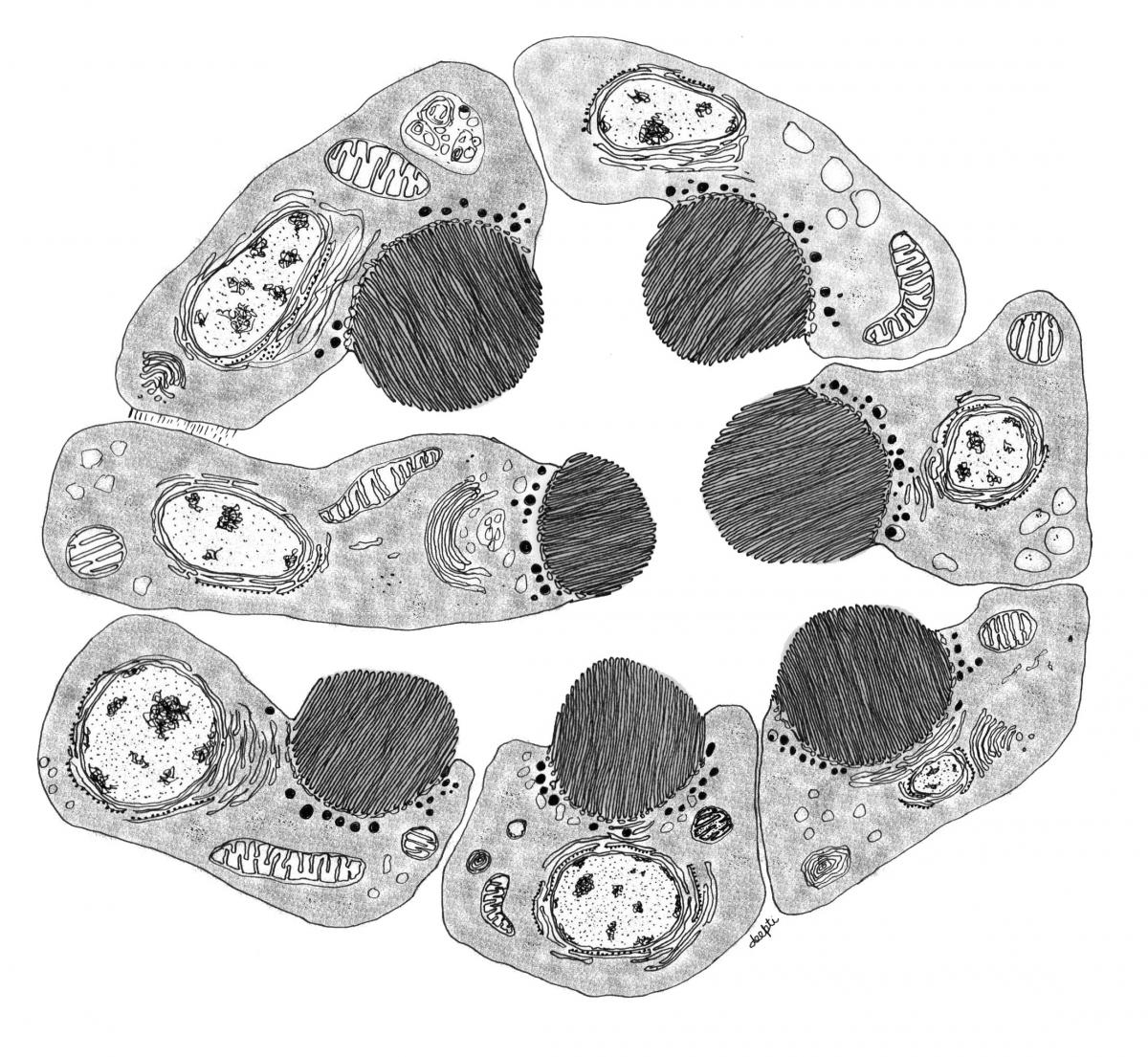
Sub-cellular organization in neurons
Rapid signaling is critical to neuronal function and given these are large cells localization and compartmentalization of molecules and biochemical reactions is key to achieving speed and specificity. In the context if lipid molecules, this requires the rapid transfer of lipid signaling intermediates between cellular organelles. This is thought to occur via membrane contact sites, sites within cells where two organelle membranes come in close proximity without fusing. We study the regulation of contact site structure and function using Drosophila photoreceptors where lipid transfer between the plasma membrane and endoplasmic reticulum is essential for supporting PI(4,5)P2 turnover during PLC signaling. The work is done using a combination of lipid biochemistry, molecular genetics, electron microscopy and in vivo imaging. Selected Publications- Cockcroft S, Raghu P. Phospholipid transport protein function at organelle contact sites. Curr Opin Cell Biol. 2018 Aug; 53:52-60. doi: 10.1016/j.ceb.2018.04.011. Epub 2018 May 30.
- The Drosophila photoreceptor as a model system for studying signalling at membrane contact sites. Yadav S, Cockcroft S and Raghu P*. Biochem Soc Trans. (2016) Apr 15;44(2):447-51. doi: 10.1042/BST20150256
- Yadav, S., Garner, K., Georgiev, P., Li, M., Gomez Espinosa, E., Panda, A., Mathre, M., Cockcroft, S and Raghu, P. RDGBα, a PI-PA transfer protein, regulates G-protein-coupled PI(4,5)P2 signalling during Drosophila phototransduction. J.Cell.Sci. 2015; 128:3330-3344
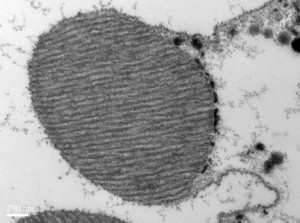
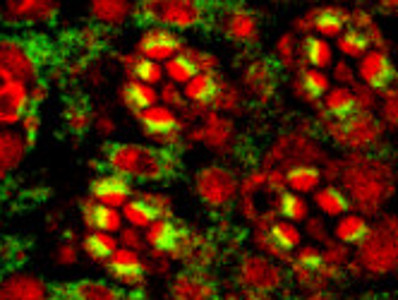
Regulation of membrane trafficking by phosphoinositides
In addition to their role in supporting receptor mediated signaling, phosphoinositides are key regulators of membrane trafficking and cellular organization. We study this problem using both Drosophila photoreceptors and iPSC derived neural models in culture. The function of phosphatidylinositol 5 phosphate 4-kinase its substrate PI5P and product PI(4,5)P2 is being studied using both the Drosophila model as well as the human genetic disorder Lowes syndrome where patients harbor mutations in the PI(4,5)P2 5-phosphatase OCRL. We are also studying the functions of PIP4K in regulating membrane transport.
- Sharma S, Mathre S, Ramya V, Shinde S, Raghu P. Phosphatidylinositol 5 Phosphate 4-Kinase Regulates Plasma-Membrane PIP3 Turnover and Insulin Signaling. Cell Rep. 2019 May 14;27(7):1991-2001.e5. doi: 10.1016/j.celrep.2019.04.070
- Kamalesh K, Trivedi D, Toscano S, Kolay, S, Sharma, S and Raghu P. Phosphatidylinositol 5- phosphate 4-kinase regulates clathrin mediated endocytosis in developing Drosophila photoreceptors J. Cell. Sci. 2017; 130: 2119-2133; doi: 10.1242/jcs.202259
- Gupta, A, Sarah Toscano, S, Trivedi, D, Jones DJ, Mathre S, Clarke J, Georgiev P, Divecha N and Raghu P. (2013) Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signalling and cell growth during Drosophila development. Proc.Natl.Acad.Sci.USA published ahead of print March 25, 2013, doi:10.1073/pnas.1219333110).
- Thakur RS, Panda A, Coessens E, Raj N, Yadav S, Balakrishnan S, Zhang Q, Georgiev P, Basak B, Pasricha R, Wakelam MJO, Ktistakis N and Raghu P. Phospholipase D activity couples plasma membrane endocytosis with retromer dependent recycling. eLife. 2016 5:e18515. DOI: 10.7554/eLife.18515.